- Why do we care about minerals and rocks?
- They contain clues about Earth in the past
- They record past processes
- They are an important resource and used in everyday life (e.g. silicon in processors)
How do we define a mineral and a rock?
- Mineral
- Solid
- Natural
- Inorganic
- Ordered internal structure
- Specific chemical composition
- Characteristics to distinguish minerals
- Crystal form
- How a mineral would grow unencumbered by space and with enough atoms etc.
- Cleavage vs No cleavage
- Has to do with the way it breaks, which also has to do with the way the molecules are bonded
- Color
- Color of the mineral itself
- Luster
- Hardness
- Is a scale
- Effervescence
- Some minerals will dissolve from acid like Calcite
- Streak
- Color of the “shavings” when you scrape the mineral against a plate
- Magnetism
- Density
- Crystal form
- Theres well over 3800 minerals that exist, but only a few are important for forming rock
- Olivine, Pyroxene, Amphibole, Plagioclase Feldspar, Biotite, Quartz, Muscovite, Potassium Feldspar (K-spar)
- These make up the vast majority of rocks and things in the mantle/crust
- Olivine, Pyroxene, Amphibole, Plagioclase Feldspar, Biotite, Quartz, Muscovite, Potassium Feldspar (K-spar)
- Rock
- Naturally formed
- Consolidated material composed of grains of one or more minerals
- It can be made of just one mineral, in that case depending on the context you may call it a rock. Usually it’s more than one mineral.
- Things we can use when describing rocks

- Color
- Grain size
- Grain shape
- How the grains interact
- The different types of grains
- More than one mineral makes this a crystalline (igneous) rock
- Igneous vs Sedimentary

- The left is Igneous and Crystalline, it solidified from a melt
- The right is sedimentary and Clastic. It is made up of cemented grains or clasts of other rocks
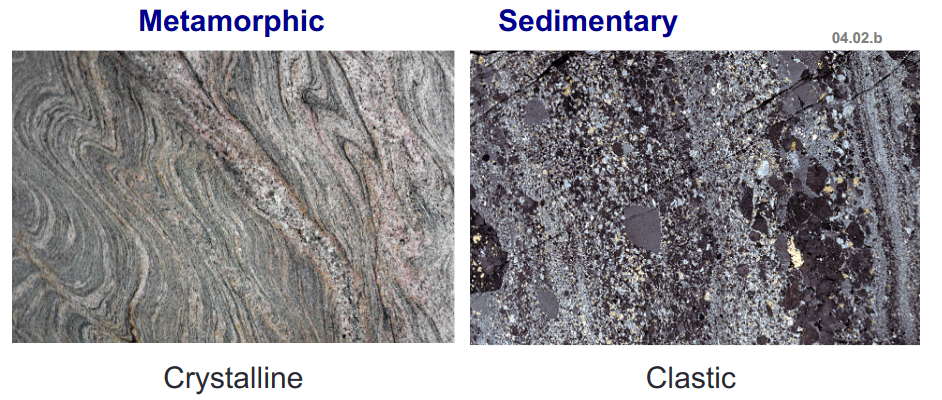
- We also have metamorphic rocks. Metamorphic rocks also form similar to igneous rocks, but they didn’t just melt and resolidify. They instead folded without melting due to high temperature and pressure. They are often the result of ductile folds.

- Three main rock types, with three different ways of forming
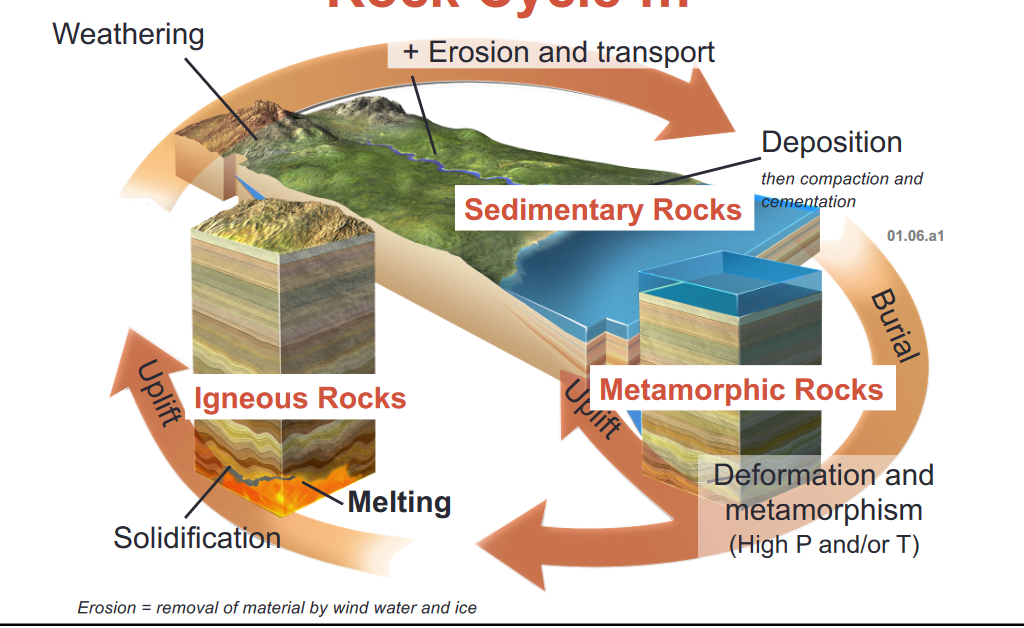
- This is the Rock Cycle
- Major compositional classes of crustal rocks
- Classified by amount of silica
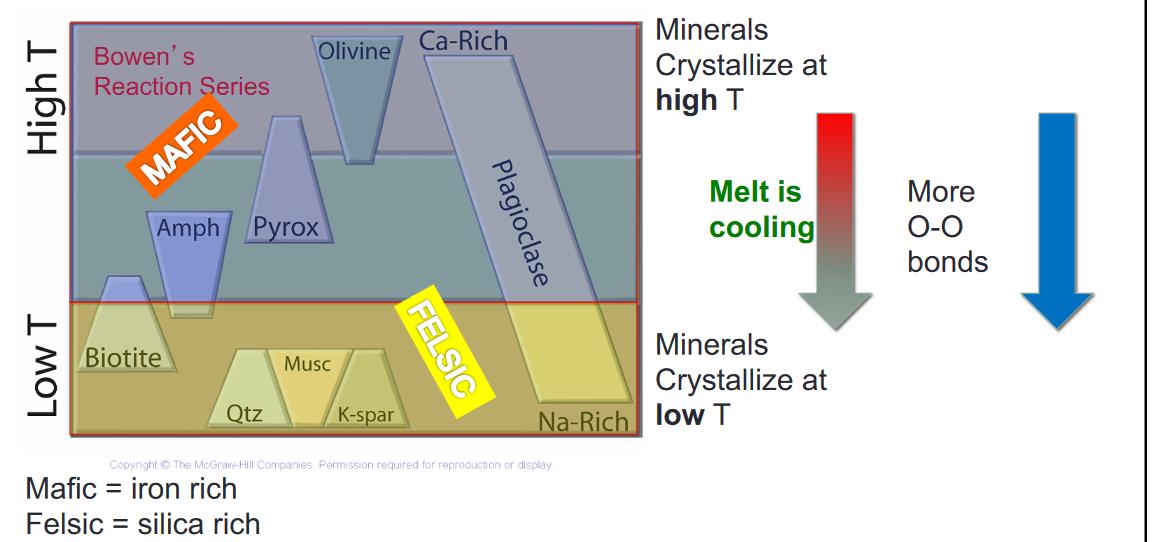
- Mafic = iron rich
- Magnesium and Iron (F is its chemical symbol)
- Felsic = silica rich
- Feldspar and Silica
Compositional layers - minerals in different parts of the earth

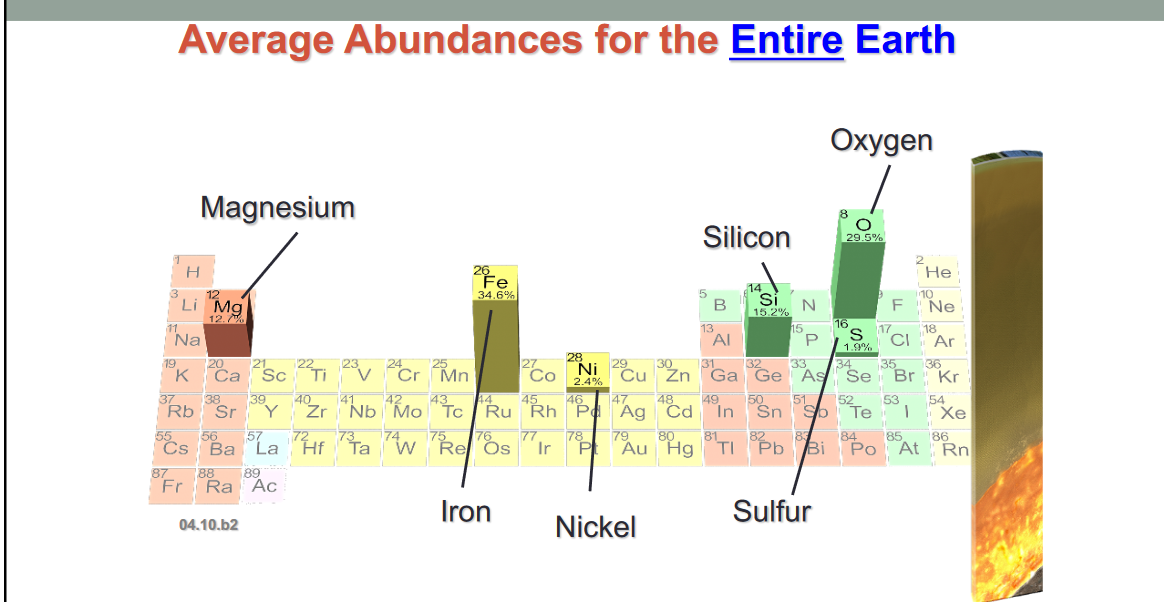
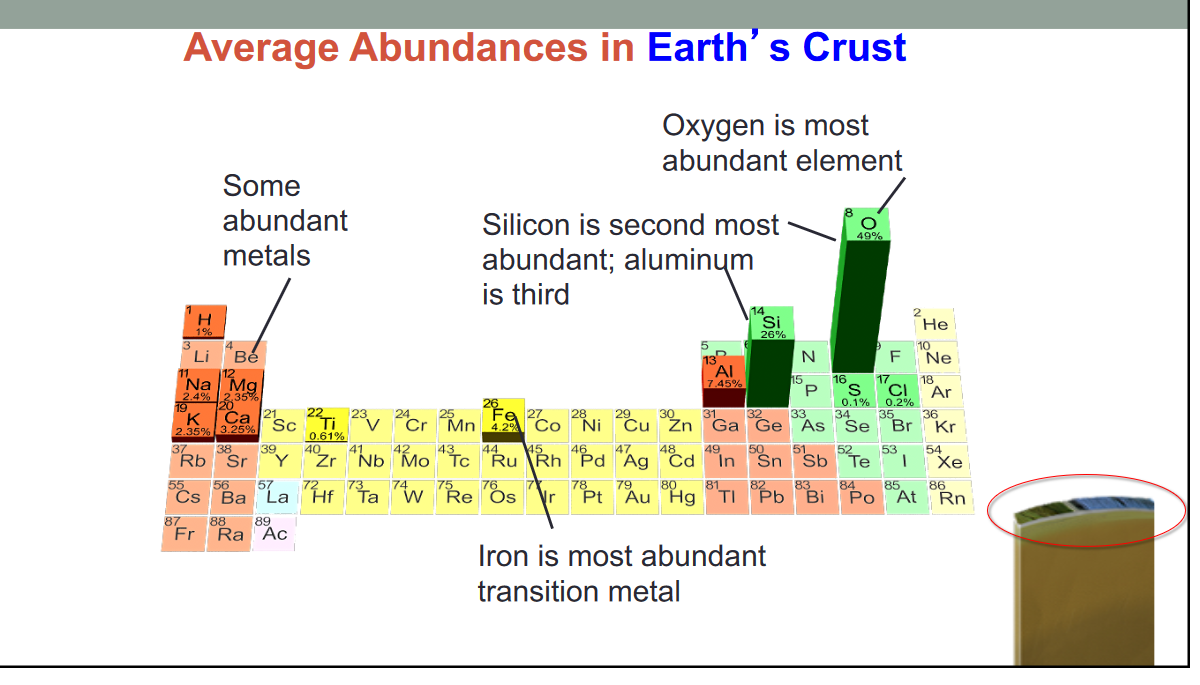
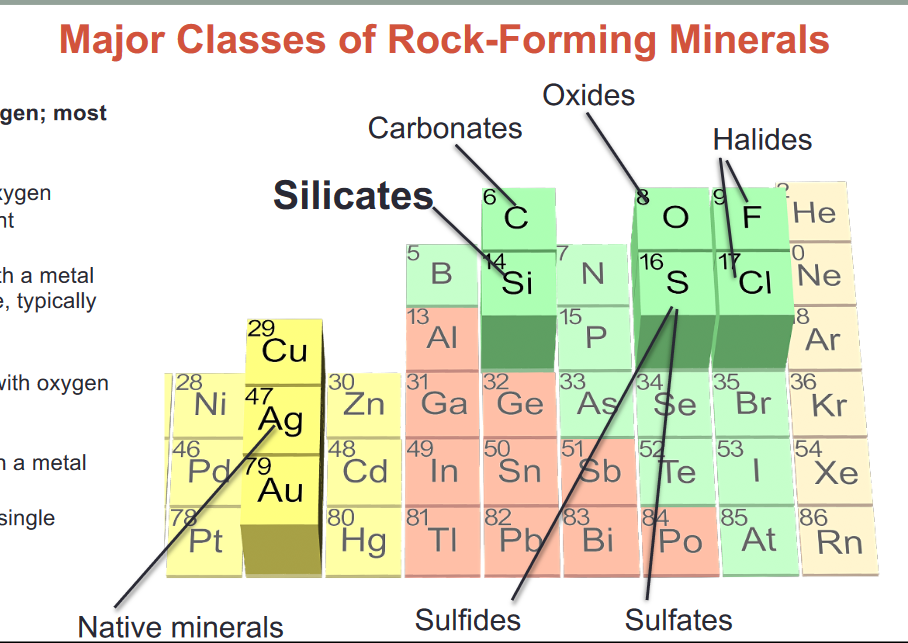
- Major classes of rock forming materials
- Silicates: Silicon and oxygen; most common
- Carbonates: carbon and oxygen bonded with another element
- Oxides: Oxygen bonded with a metal
- Halides: Chlorine or Fluorine, typically bonded with a metal
- Sulfates: Sulfur combined with Oxygen and bonded to a metal
- Sulfides: Sulfur bonded with a metal
- Native minerals: contain a single element
Silicate Tetrahedron
- The key rock forming minerals are all silicates
- These silicates can be described by Bowen’s Reaction Series: idealized progression of how key minerals crystalize in a cooling melt
- Silicate Tetrahedron - Building block for major rock forming minerals
- Silicon in the center with Covalent bonds with Oxygen
- Tetrahedra bond together and to other elements
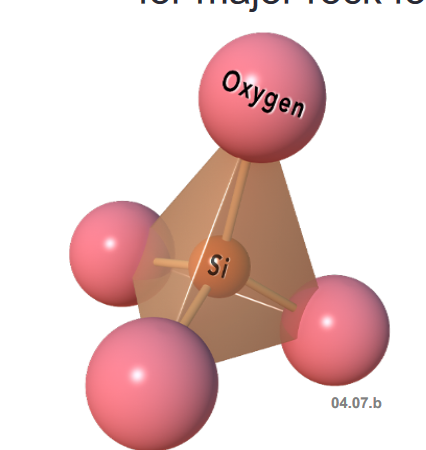
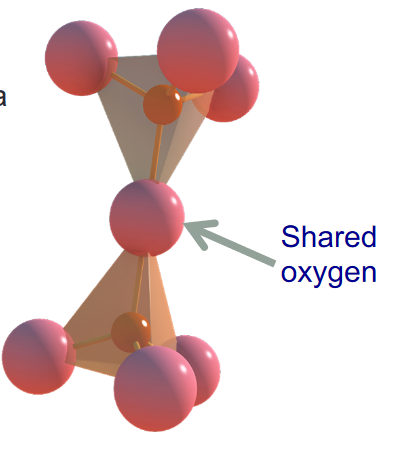
- Silicon and Oxygen combine to form the basic silica tetrahedron, which is the fundamental building block of rock material
- A silica tetrahedron is not electrically neutral
- It needs to bond with something, usually metals
- The more silica (or less metal ions) present in a mineral structure, the more silica needs to bond to itself to balance charge
- The classes of crustal rocks
- Felsic: A rock or melt with relatively HIGH silica
- Crystallize = Melt at low temperature in Bowen’s Reaction Series
- Intermediate
- Mafic: A rock or melt with relatively LOW silica
- Crystalize = Melt at high temperature in Bowen’s Reaction Series
- Felsic: A rock or melt with relatively HIGH silica
- Fractional Crystallization
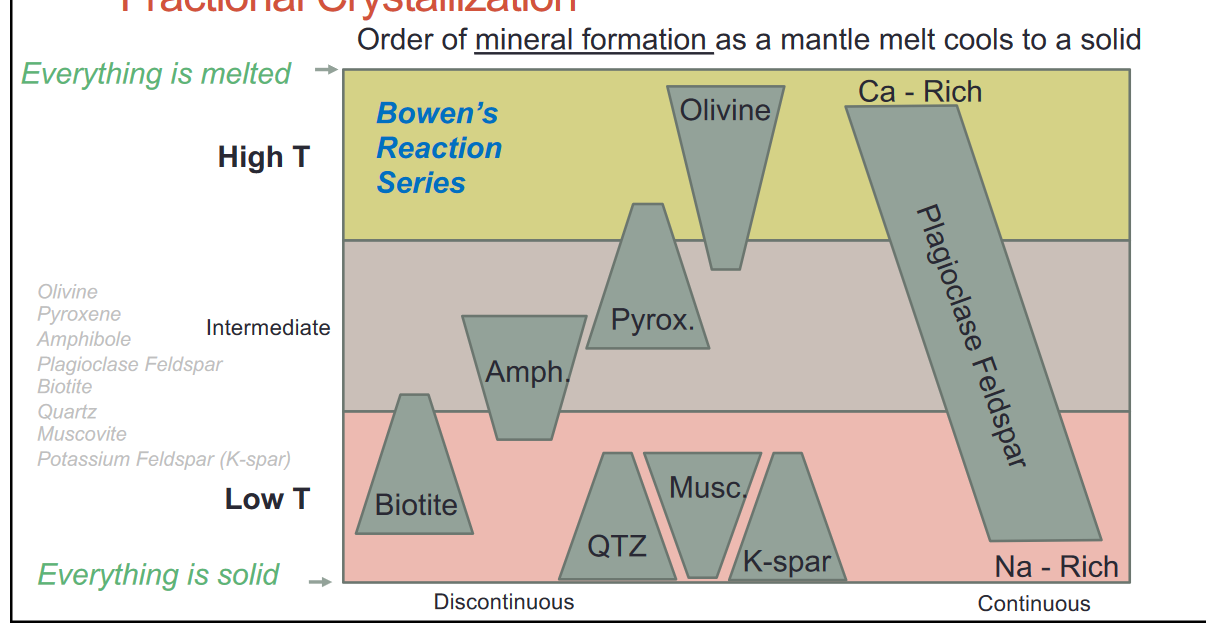
- As we go down there are more O-O bonds
- The top has more ionic bonds the bottom has more covalent bonds
- Top is Mafic, bottom is Felsic
Structure of Silicate Minerals
- Cleavage: Internal structure influences external form
- Cleavage in a mineral means theres preferred planes for breaking within the mineral, due to the alignment of bonds
- No preferred planes means theres similar strength bonds in all directions